Heep Woh College
F.6 Reading
Assignment 2
Marking Scheme
02-I-4
 (a) liquid 1
(a) liquid 1
(b)
1
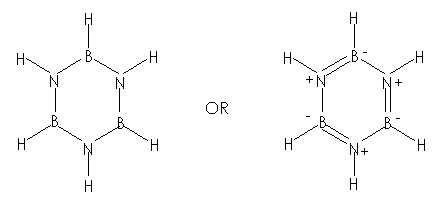
Borazine is a conjugated
system. The p-electrons
are delocalized. ½
The molecule is
symmetrical 1
Therefore, the molecule
does not possess a net dipole moment. ½
(c) (i) In
benzene, the C¾C
bonds are non-polar. ½
In
borazine, the B¾N
bonds are polar (as N is more electronegative than B)/ the N atoms are
negatively charged / N atoms have a relatively high p-electron density ½
N
atoms in borazine are more susceptible to electrophilic attack.
OR B atoms in borazine are more susceptible to nucleophilic
attack.
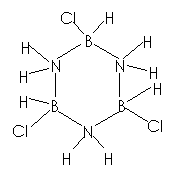
(ii)
1
(d)
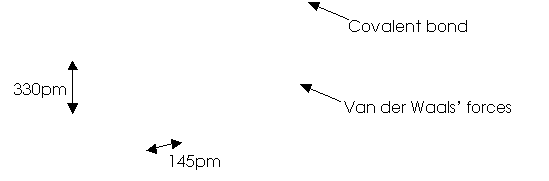
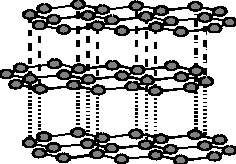
3
(1 mark
for the structure; 1 mark for labeling B¾N bond distance and distance
between two layers; 1 mark for labeling the types of interaction.)
(e) In (BN)x there is much less
delocalization of p-electrons
(since the boron and nitrogen 2p orbitals do not contribute equally to the
delocalized p-system). 1